Participants Information Leaflet
Dear participant,
We would like to invite you to participate in a study that reveals the connection between parameters of our bipedal locomotion and pelvic girdle architecture.
Your participation in this study is voluntary. You can refuse to participate at any time, without having to give a reason, or also withdraw your agreement to participate once the study has already started. There will be no negative consequences for you if you refuse to participate or if you withdraw from this study early.
This kind of study is necessary to gain new, reliable academic and research results. However, your written consent to participate in the study is an indispensable prerequisite for us to conduct this study. Please take time to read the following information carefully, and do not hesitate to ask questions.
Please only sign the declaration of consent if you have fully understood the type and procedure of the study, if you are willing to give your consent to participate, and if you are aware of your rights as a participant in this study.
1. What is the purpose of this study?
This study will test if the structure of the pelvic girdle influences biomechanical properties of upright walking. We will measure metabolic energy consumption, dynamic powers generated around each leg joint during walking and calculate joint reaction forces in the hip, i.e., the forces between the femoral head and acetabulum. These data will then be compared with the geometry of the pelvic girdle in each participant.
2. What is the procedure of the study?
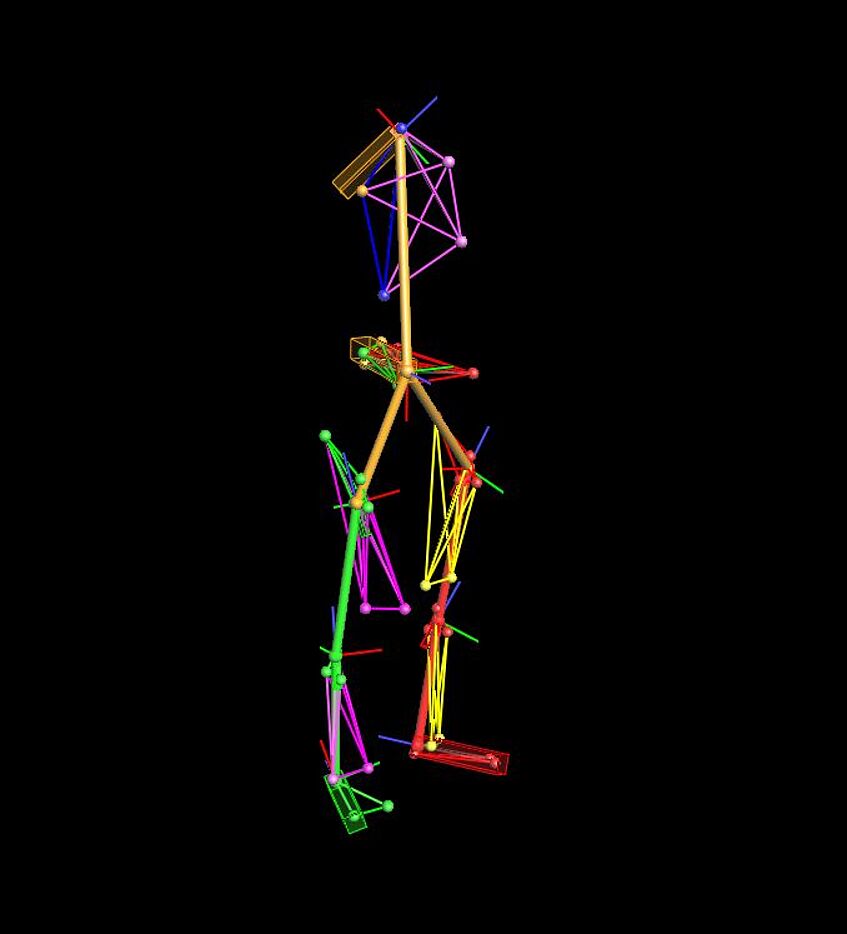
Individual Model
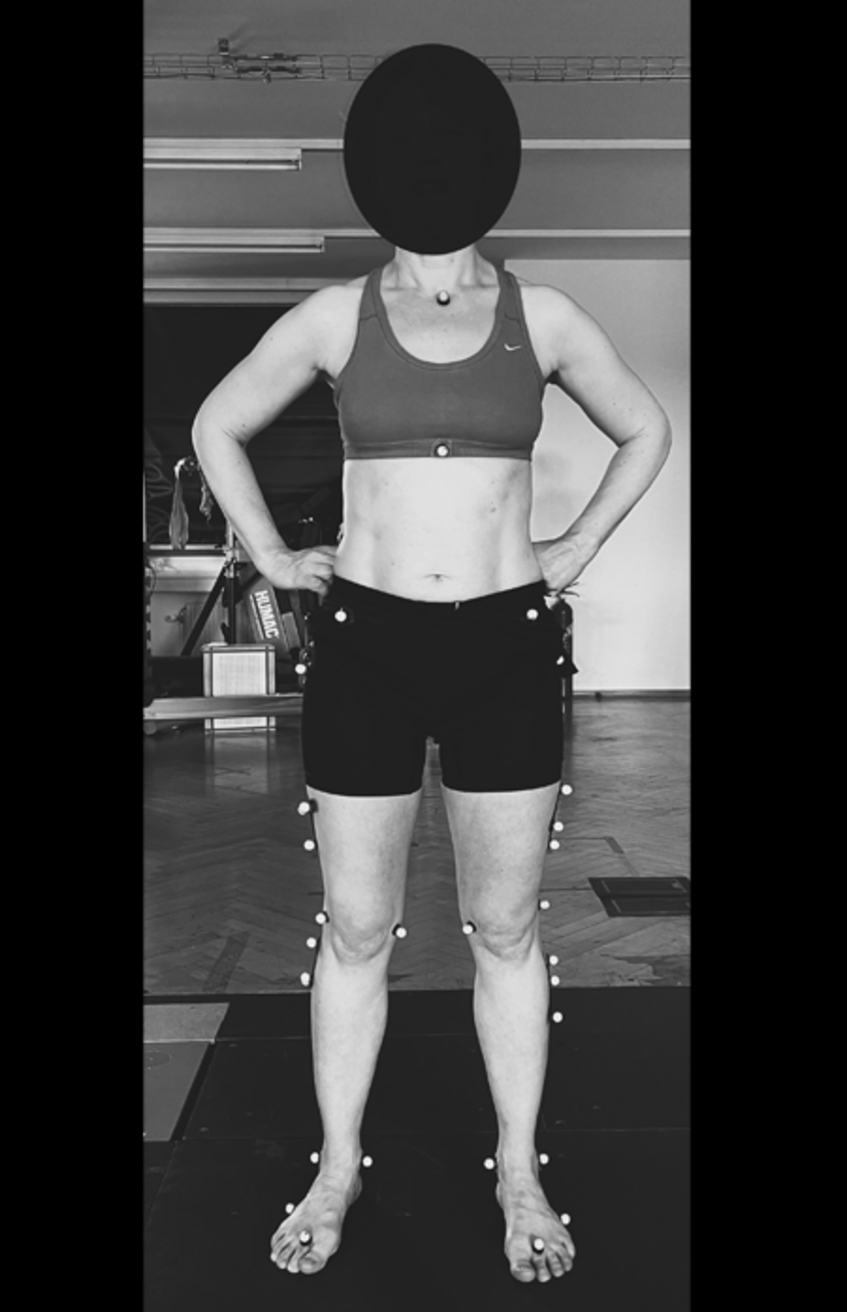
Reflective Markers
We will measure dynamic powers generated around each leg joint during walking and calculate joint reaction forces in the hip, i.e., the forces between the femoral head and acetabulum. These data will then be compared with the geometry of the pelvic girdle in each participant.
The motion recording happens at the Neuromechanics Lab of the University of Vienna:
Auf der Schmelz 6a (USZ II)
1150 Wien
We aim to collect data on 50 healthy volunteers, 25 men and 25 women. The participants will need to
- Take part in the motion analysis at the kinematic laboratory of the Institute for Biomechanics, University of Vienna; and
- Undergo a magnetic resonance (MR) imaging of the lower limbs and pelvis at the Orthopaedic Hospital Speising.
You can participate in the study if you are
- Age 18-65
- Have no congenital disorders
- Have had no orthopaedic surgeries, planned or completed
- Have no metallic implants or plates
- Have no pacemaker, cochlear implant or defibrillator
- Preferably, have no tattoo as tattoo ink may contain ferromagnetic metals and create irritation and/or magnetic artefacts.
- Can walk unassisted
- Have BMI (body mass index is mass in kg divided by squared height in m) in the range of 18 to 30
- Not pregnant
The motion of lower limbs and pelvic girdle during walking will be recorded with the help of force plates, EMG sensors and a camera-based movement analysis system (VICON). For precise motion detection it is necessary to position reflective markers to trace motion at different points of lower extremities. Only the movements of the lower extremities are recorded, but no videos or images of the entire person are recorded.
We recommend that the volunteers wear tight sports clothes, with shorts rather than long trousers or leggins. The time required per participant is around 2 hours.
The MR images will be taken at the at the Orthopedic Hospital Speising Vienna:
Orthopädisches Spital Speising
Speisinger Straße 109
(Linie 60, Station Riedelgasse)
1130 Wien
3 Tesla “Magnetom Sola” (Siemens) uses a magnetic field and a computer to crate detailed slices of your pelvis and hip area. It allows for detailed representation of your bone and muscle attachments at the hip. The time required per participant is around 60 minutes.
3. What are the benefits of participating in the study?
The participants will make an important contribution to a better understanding of the relationship between the architecture of the pelvic girdle and biomechanical parameters of walking. In principle, the participants cannot derive any direct benefit from participating in the study. However, on request or if there is personal interest, the participants will receive their EMG or force measurement data and a radiology report of examined regions. The time and travel will be reimbursed by a set amount of 50 euros at the end of the second test.
4. What are the possible risks of taking part this study? Could participants experience any discomfort or other side effects?
There are no risks associated with participating in motion data collection (Part 1). All exercises to be recorded are safe and none of the measuring instruments pose a risk of injury. While the markers are being attached, the recommended minimum distance to protect against further spread of the corona virus cannot be maintained. However, all possible protective measures (hand disinfection, FPP2 masks) will be used to keep possible transmission as low as possible. In addition, the test is only carried out if the test subjects and test managers are completely healthy (no flu-like symptoms).
Part 2 involves magnetic resonance (MR) imaging of the lower body. MR imaging is very safe, and most people can have the procedure. However, it is not recommended if you have an implant from a ferromagnetic material (iron, nickel, cobalt) or an implanted pacemaker.
5. Does participating in the study have any other effects on participants’ lifestyle? What are the obligations resulting from participating?
Apart from the described direct experimental procedure of the study (see point 2), neither effects on the way of life are to be expected, nor are there any obligations after participation
6. What should participants do if they experience symptoms of complaints, unwanted side effects and/or injuries?
Participation in the study can be cancelled at any time if pain or discomfort occurs. To do this, please get in touch with the test manager or the principal investigator (see point 10).
7. In what cases is it necessary that participants withdraw from the study early?
Participants can withdraw their consent to participate in the study at any time and without giving reasons. You can also withdraw from the study for personal reasons without this being detrimental to you.
If, once enrolled into the study, the participant can no longer meet criteria listed in point 2, the early withdrawal is recommended. Please, inform the principal investigator (see point 10).
The principal investigator can decide to end a person's participation in the study. Possible reasons for this can be:
a) The participant cannot meet the requirements of the study.
b) The principal investigator has the impression that further participation in the study is not in the interest of the participant
8. How will the data collected in this study be used?
The data collected as part of the study are stored and processed in a pseudonymised form, i.e., provided with an identification key (ID number). Any further processing and analysis of the collected data is only carried out on the basis of this ID number. Only test managers and the principal investigator know the connection between identity and ID number and are obliged to maintain confidentiality. This information is stored on an encrypted external hard drive in a locked cabinet in the principal investigator’s office.
Data is only processed and passed on for purposes of biomechanical modelling and statistical analysis. Only the staff involved in the study have access to the data and are bound to secrecy. The names of the participants are also not mentioned in any publications of the data from this study and no conclusions can be drawn about the participants.
Participants can request the deletion of their data up to 12 months after the data entry without giving reasons by personal, telephone or written notification to the study management. The identification key is deleted twelve months after the data have been collected, so the data are anonymised from this point on and can no longer be assigned to individual test subjects. From this point on, your data can no longer be assigned and therefore can no longer be deleted. The anonymised data (data including ID number without personal data) are stored on an NAS server and are password-protected and accessible for the study staff.
9. Will there be any costs for the participants? Will they receive reimbursement or remuneration?
You will not incur any costs for participating in this study, the participants will be remunerated for their time and travel in the fixed amount of 50 euros on the completion of both tests.
10. Possibility to discuss further questions.
The study coordinator will be happy to answer any further questions during or after participation.
Name: Katya Stansfield
E-mail: katya.stansfield@univie.ac.at
Phone: +44 777 1962137
